Our research in
Peptidomimetic chemistry
Functions fulfilled by proteins depend to a large extent on the ability of the intrinsically flexible polypeptide chain to fold correctly into well-ordered and compact tertiary structures and eventually to (self-)assemble. Multiple approaches, at the interface between biology, synthetic organic and polymer chemistries are currently being developed to elaborate synthetic systems with protein-like structures and functions.
By using peptidomimetic chemistry, the general aims of our research are (i) to understand how to program molecules with the necessary information for self-ordering into complex and functional architectures, (ii) to create folded systems mimicking protein secondary structure elements (e.g. helices), (iii) to study interactions with biomolecules and to develop biomedical applications.
Foldamer chemistry
Synthesis of folded and self-assembled architectures mimicking proteins
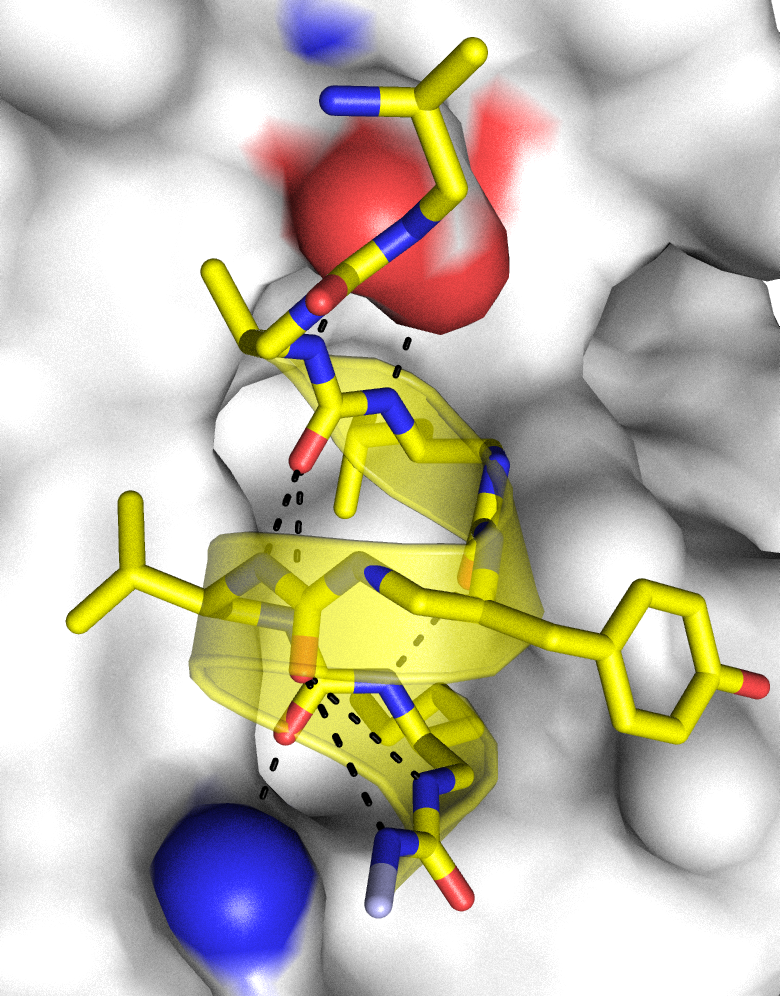
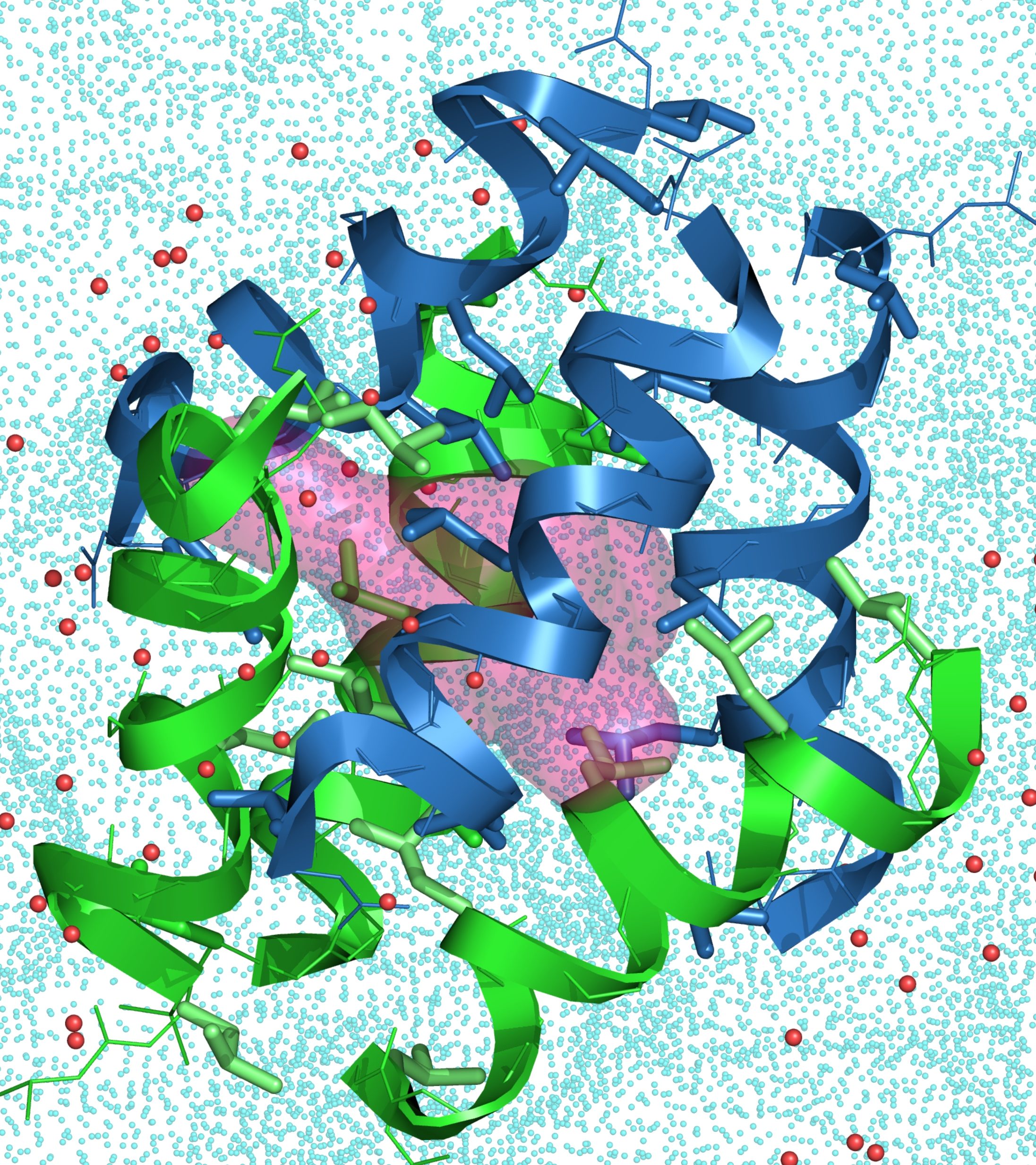
One of the main line of research in the Guichard group is the construction of new synthetic folded systems mimicking biopolymers (‘foldamers’) coupled to the exploration of the interplay between folding and molecular recognition properties. Using proteins as inspiration, our group has pioneered the development of peptidomimetic foldamers without amino acids by showing that aliphatic N,N’-linked urea oligomers form well defined and remarkably stable helical secondary structures reminiscent of the α-helix. Our current projects focus on the development of more sophisticated and functional architectures including peptide/oligourea hybrids, the formation of new folding patterns, the design of composite proteins (tertiary structures), and of nanostructures formed by controlled foldamer assemblies (quaternary structures).
Chemical biology and medicinal chemistry
From protein surface recognition to peptide therapeutics
Peptides are useful molecules to interfere with biomolecular interactions, investigate biological processes and develop new modalities for therapeutic applications. In the group, we are combining peptidomimetic and foldamer chemistries, structure-guided design and chemical biology to develop effective ligands against targets of various therapeutic interest (receptor ligands, disruptors of protein-protein interactions). In particular, we are interested in approaches to constrain peptides as they may yield compounds with unusual activity profiles including improved binding, higher resistance to degradation by proteases, and cell permeability compared with unconstrained peptides.
Molecular Recognition and Catalysis
Synthesis of molecules with unique recognition properties
The ability to synthesize sequence-based non-natural oligomers that fold with high fidelity bodes well for creating molecules with unique recognition properties tailored to various applications (e.g. catalysis, sensing). We are also interested in molecular recognition properties of foldamer-based quaternary and tertiary structures, as supramolecular cavities and channels generated from short foldamer sequences may provide privileged environments for interaction with a broad range of guest molecules.